

|
姓 名: |
杨凯 |
性 别: |
男 |
|
职 称: |
副研究员/博士生导师 |
|
学 历: |
博士 |
|
电 话: |
||
专 业: |
有机化学 |
|
电子邮件: |
kyang@fzu.edu.cn |
|
研究方向: |
有机硼化学 |
![]() 教育工作经历
教育工作经历
2020.11~至今 学院,皇冠国际体育app,副研究员
2018.09~2020.10 学院,皇冠国际体育app,博士后
2013.09~2018.06 华侨大学,化工学院,博士
2009.09~2013.06 南华大学,药学院,学士
![]() 教学简介
教学简介
![]() 科研简介
科研简介
致力于发展高效、高选择性和普适的方法合成手性有机硼化合物,并探索这类化合物在医药、催化以及材料化学等领域的应用,以及探索硼原子的引入对反应和手性化合物性质的影响。将有机硼化学与手性化学有机结合起来,是我们未来重点关注与研究的方向。
![]() 社会兼职
社会兼职
![]() 科研项目
科研项目
(1) 国家自然科学基金面上项目(2023-2026)
(2) 国家自然科学基金青年基金(2021-2023)
(3) 福建省自然科学基金青创项目(2022-2025)
![]() 代表性论文
代表性论文
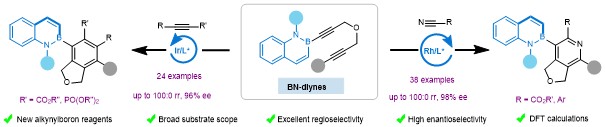
20.Wang, H.; Qiao, B.; Zhu, J.; Guo, H.; Zhang, Z.; Yang, K.*; Li, S.-J.; Lan, Y.*; Song, Q.* Enantio- and Regioselective [2 + 2 + 2] Cycloaddition of BN-Diynes for Construction of C-B Axial Chirality. Chem 2023, DOI: 10.1016/j.chempr.2023.09.017.
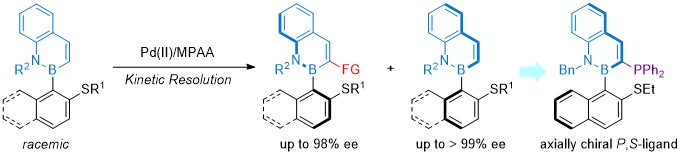
19.Xu, J.; Qiu, W.; Zhang, X.; Wu, Z.; Zhang, Z.; Yang, K.*; Song, Q.* Palladium‐Catalyzed Atroposelective Kinetic C−H Olefination and Allylation for the Synthesis of C−B Axial Chirality. Angew. Chem. Int. Ed. 2023, 62, e202313388.
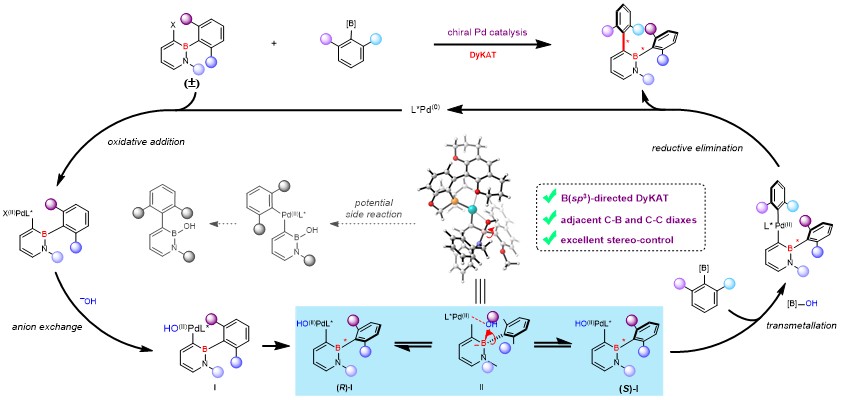
18. Yang, K.# Mao,Y.# Zhang, Z.# Xu, J.; Wang, H.; He,Y.; Yu, P.*; Song, Q.* Construction of C-B axial chirality via dynamic kinetic asymmetric cross-coupling mediated by tetracoordinate boron, Nat. Commun. 2023, 14, 4438.
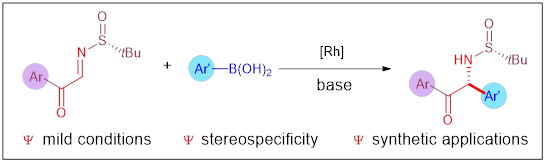
17. Wang, C.; Zhou, L.; Qiu, J.; Yang, K.*; Song, Q.* Rh-Catalyzed diastereoselective addition of arylboronic acids to α-keto N-tert-butanesulfinyl aldimines: synthesis of α-amino ketones. Org. Chem. Front. 2022, 9, 1016-1022.
16. Xu, H.; Ye, M.; Yang, K.*; Song, Q.* Regioselective Cross-Coupling of Isatogens with Boronic Acids to Construct 2,2-Disubstituted Indolin-3-one Derivatives. Org. Lett. 2021, 23, 7776-7780.

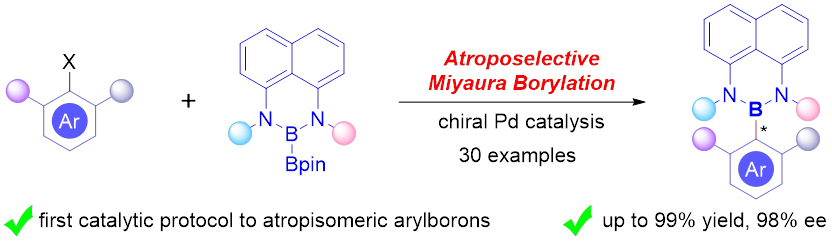
15. Yang, K.; Mao, Y.; Xu. J.; He, Y.; Li, W.; Song, Q.* Construction of axially chiral arylborons via atroposelective miyaura borylation. J. Am. Chem. Soc. 2021, 143, 10048-10053.
14. Yang, K.; Song, Q.* Tetracoordinate boron intermediates enable unconventional transformations. Acc. Chem. Res. 2021, 54, 2298-2312.
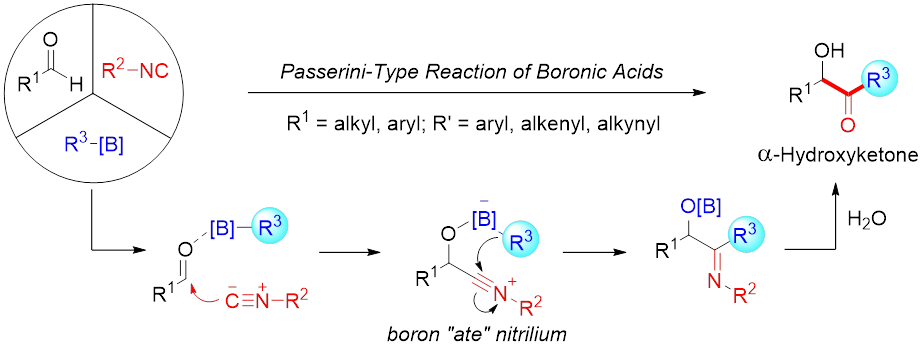
13. Yang, K.; Zhang, F.; Fang, T.; Li, C.; Li, W.; Song, Q.* Passerini-type reaction of boronic acids enables α-hydroxyketones synthesis. Nat. Commun. 2021, 12, 441.
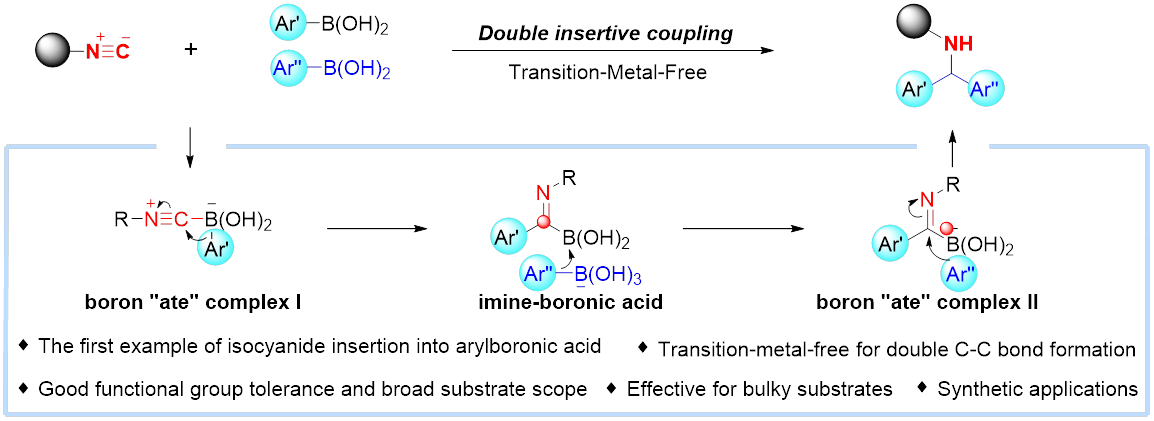
12. Yang, K.; Hu, X., Li, W.; Qiu, J.; Feng, Q.; Wang, S.; Zhang, G.; Kuang, Z.; Yu, P.*; Song, Q.* Transition-metal-free double insertive coupling of isocyanides with arylboronic acids enabled diarylmethanamines. Cell Rep. Phys. Sci. 2020, 1, 100268.
11. Yang, K.; Kuang, Z.; Song, Q.* Radical-induced 1,2-boron shift, enabling 1,3-difunctionalization of allylboronic esters. Chem, 2020, 6, 330-331.
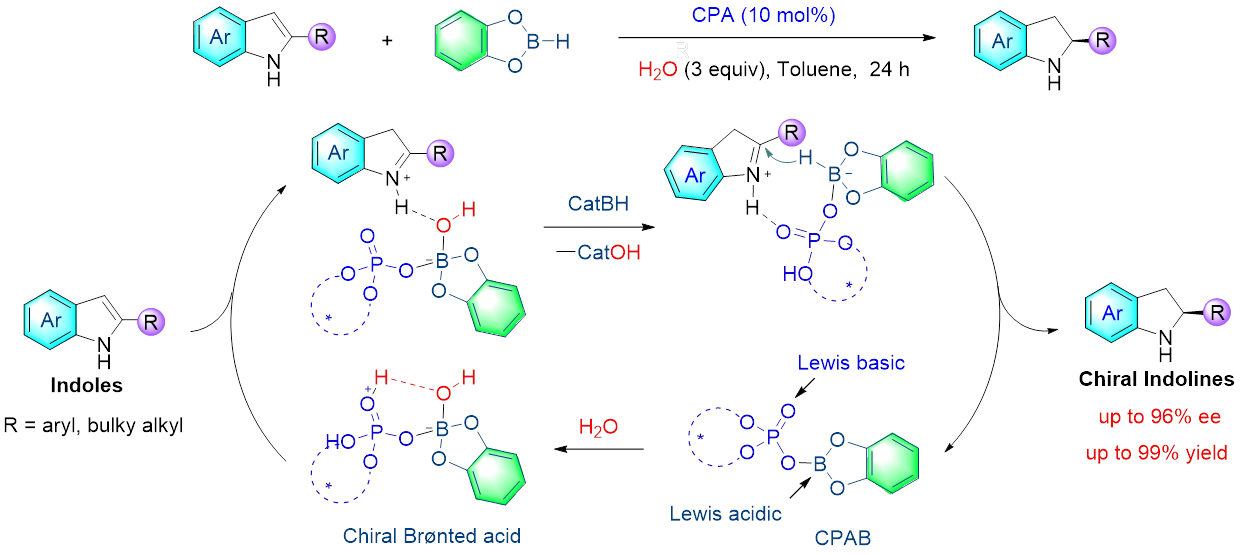
10. Yang, K.; Lou, Y.; Wang, C.; Qi, L.-W.; Fang, T.; Zhang, F.; Xu, H.; Zhou, L.; Li, W.; Zhang, G.; Yu, P.*; Song, Q.* Chiral brønsted acid from chiral phosphoric acid boron complex and water: asymmetric reduction of indoles. Angew. Chem. Int. Ed. 2020, 59, 3294-3299.
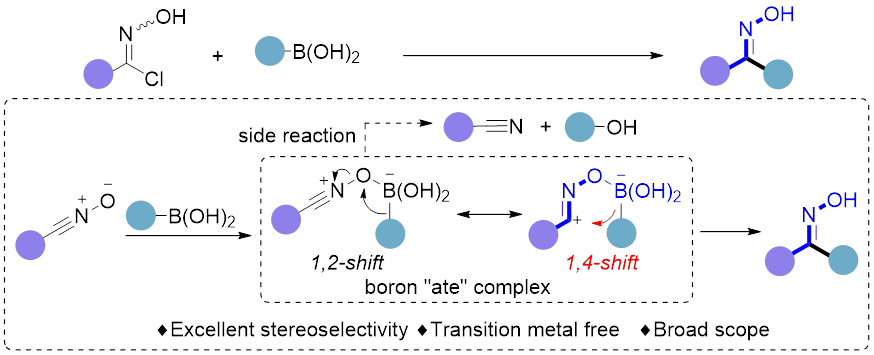
9. Yang, K.; Zhang, F.; Fang, T.; Zhang, G.; Song, Q.* Stereospecific 1,4-metallate shift enables stereoconvergent synthesis of ketoximes. Angew. Chem. Int. Ed. 2019, 58, 13421–13426.
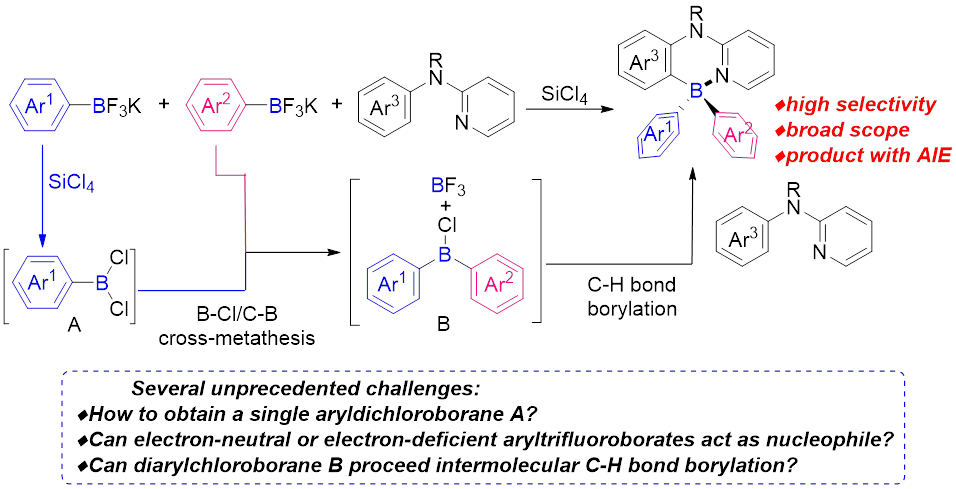
8. Yang, K.; Zhang, G.; Song, Q.* Four-coordinate triarylborane synthesis via cascade B–Cl/C–B cross-metathesis and C–H bond borylation. Chem. Sci. 2018, 9, 7666-7672.
7. Yang, K.; Song, Q.* Transition-metal-free regioselective synthesis of alkylboronates from arylacetylenes and vinyl arenes. Green Chem. 2016, 18, 932-936.
6. Yang, K.; Ke, M.; Lin, Y.; Song, Q.* Sulfonamide formation from sodium sulfinates and amines or ammonia under metal-free conditions at ambient temperature. Green Chem. 2015, 17, 1395-1399.
5. Yang, K.; Song, Q.* Pd-Catalyzed Regioselective Arylboration of Vinylarenes. Org. Lett. 2016, 18, 5460−5463.
4. Yang, K.; Song, Q.*. Fe-Catalyzed Double Cross-Dehydrogenative Coupling of 1,3-Dicarbonyl Compounds and Arylmethanes Org. Lett. 2015, 17, 548-551.
3. Yang, K.; Zhou, F.; Kuang, Z.; Gao, G.; Driver, T. G.; Song, Q.* Diborane-Mediated Deoxygenation of o‑Nitrostyrenes To Form Indoles. Org. Lett. 2016, 18, 4088-4091.
2. Yang, K.; Song, Q.* Palladium-Catalyzed Arylboration of Bicyclic Alkenes. J. Org. Chem. 2016, 81, 1000-1005.
1. Yang, K.; Song, Q.* Styryl ether formation from benzyl alcohols under transition-metal-free basic DMSO conditions. Org. Biomol. Chem. 2015, 13, 2267-2272.
![]() 其他
其他